vanadium electronic configuration|v electronic configuration : Tuguegarao Electron configuration 3d 3 4s 2: Electrons per shell: 2, 8, 11, 2: Physical properties; Phase at STP: solid: Melting point: 2183 K (1910 °C, 3470 °F) Boiling point: 3680 K (3407 °C, 6165 °F) Density (near .
Get menu, photos and location information for Village Squire Of Mchenry in McHenry, IL. Or book now at one of our other 14628 great restaurants in McHenry.
PH0 · vanadium valency
PH1 · v electronic configuration
PH2 · Iba pa
If you are looking for custom-made high visibility and durable sign same as these DOTr Directory Signs? Call us today! Related Posts: Outdoor Hospital Directional Sign June 13, 2024. DOTr Outdoor Building Directory Sign Upd. June 24, 2024. Camaya Coast Directional Sign June 13, 2024.
vanadium electronic configuration*******March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.Click on above elements (in Periodic table) to see their information or Visit .
The electronic configuration of Vanadium can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d3. Vanadium has an atomic number of 23, which means it has 23 .
The Electron configuration of vanadium is [Ar]3d34s2. Vanadium is defined as a chemical element belonging to the periodic table, it is part of group 5, it is represented by the . To write the configuration for the Vanadium and the Vanadium ion, first we need to write the electron configuration for just Vanadium (V). We first need to find the number of electrons.Electron configuration 3d 3 4s 2: Electrons per shell: 2, 8, 11, 2: Physical properties; Phase at STP: solid: Melting point: 2183 K (1910 °C, 3470 °F) Boiling point: 3680 K (3407 °C, 6165 °F) Density (near .Vanadium, electron configuration. Periodic table » Vanadium » Electron configuration. Vanadium. Full electron configuration of vanadium: 1s2 2s2 2p6 3s2 3p6 3d3 4s2. .vanadium electronic configurationElectron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
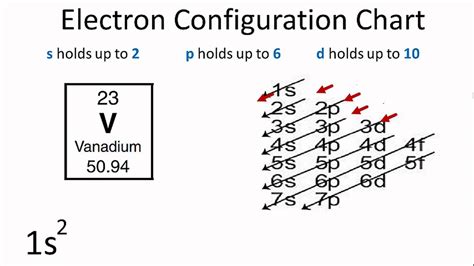
We can write the electron configuration of vanadium using four different methods: #1 Using aufbau principle. #2 Using periodic table. #3 From its Bohr model. #4 . Vanadium Electron Configuration: Vanadium is a chemical element. It has a symbol V. The atomic number of Vanadium is 23. It is a silvery-grey, hard, malleable, and ductile transition metal. This metal is .V (Vanadium) is an element with position number 23 in the periodic table. Located in the IV period. Melting point: 1890 ℃. Density: 6.09 g/cm 3 . Electronic configuration of the Vanadium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3Electronic configuration of the Vanadium atom in ascending order of the levels . The electronic configuration of Vanadium can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d3. Vanadium has an atomic number of 23, which means it has 23 electrons in total. The electronic configuration shows how these electrons are arranged in the energy levels and sub-levels around the nucleus.La configuration électronique du vanadium est [Ar]3d34s2. Le vanadium est défini comme un élément chimique appartenant au tableau périodique, il fait partie du groupe 5, il est représenté par le symbole V et son numéro atomique est 23. C'est un .What is the electronic configuration of vanadium (V, Z=23)? SOLUTION. Vanadium is the transition metal in the fourth period and the fifth group. The noble gas preceding it is argon (Ar, Z=18), and knowing that vanadium has filled those orbitals before it, argon is used as the reference noble gas. The noble gas in the configuration is denoted E .v electronic configuration Vanadium Electron Configuration: Vanadium is a chemical element. It has a symbol V. The atomic number of Vanadium is 23. It is a silvery-grey, hard, malleable, and ductile transition metal. This metal is rarely found in . Vanadium is a chemical element with the symbol V and atomic number 23. It is a transition metal that is commonly found in nature and has various industrial applications.One of the key aspects of vanadium is its electron configuration, which determines its chemical properties and behavior. The electron configuration of an . As shown above, the Vanadium Electron Configuration of the element Vanadium is Ar 3d3 4s2. Therefore, now it is easier to understand the ground state, and the element Vanadium, its ground state is written as the following; [Ar] 3d 3 4s 2. How many must be thinking that what exactly is it, so to make it easier, the atomic number of the .Hunds Rule is called Hunds Rule of Maximum Multiplicity because the spectral lines on the left configuration of Figure 7.2.1 7.2. 1 will split into three lines in the presence of an external magnetic field. This is because for an ensemble of atoms some will have their magnetic moments perpendicular to an external magnetic field (no effect . In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .

Solution: Method 2. Locate the atom on the periodic table. Figure 1.9.1 1.9. 1: Periodic table of the elements with the location of vanadium (V) highlighted. (CC-BY-NC-SA; Kathryn A. Newton) Starting at hydrogen and the 1s subshell, read across each row of the periodic table until you get to your chosen element.
vanadium electronic configuration v electronic configurationInner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 2.6.6 2.6. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, .Electron configuration of Vanadium is [Ar] 3d3 4s2. Possible oxidation states are +2,3,4,5. The chemistry of vanadium is noteworthy for the accessibility of the four adjacent oxidation states 2–5. Vanadium (II) .
Abstract. We report on the electronic structure of vanadium in synthetic V-oxides and in natural roscoelite (V-rich phyllosilicate). This study applied electron energy-loss spectroscopy (EELS) in the scanning transmission electron microscope (STEM), combined with first-principle calculations, to (1) establish relationships between the V . Vanadium -. V: properties of free atoms. Vanadium atoms have 23 electrons and the shell structure is 2.8.11.2. The ground state electron configuration of ground state gaseous neutral vanadium is [ Ar ]. 3d3. 4s2 and the term symbol is 4F3/2. Schematic electronic configuration of vanadium. The Kossel shell structure of vanadium.
The complete electron configuration of vanadium is 1s2 2s2 2p6 3s2 3p6 4s2 3d3 . Vanadium have 5 valence electrons around the nucleus and the atomic number is 23 .The distribution of electrons is as 2 electrons in 1s subshell, 2 electrons in 2s subshell, 6 electrons in 2p subshell, 2 electrons in 3s, 6 electrons in 3p, 2 electrons in 4s and 3 . 4. Electrons always fill in the lowest energy configuration possible. Cr and Cu, as well as Cu and Ag, are exceptions in the "typical" filling order. In the case of Cr and Cu, they are stabilized by having 2 half filled orbitals, which maximizes exchange energy and minimizes electron repulsion. In their case, the energy to promote an s electron .Vanadium is a chemical element of the periodic table with chemical symbol V and atomic number 23 with an atomic weight of 50.9415 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 3: 4s 2: Electrons per shell: 2, 8, 11, 2: Valence electrons : 5: Valency electrons : 2,3,4,5:
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
This package install the Realtek PCIe GBE Family Controller Software. SHOP SUPPORT. PC Data Center Mobile: Lenovo Mobile: Motorola Smart Service Parts COMMUNITY My Account .Check out free Anime Hentai porn videos on xHamster. Watch all Anime Hentai XXX vids right now!
vanadium electronic configuration|v electronic configuration